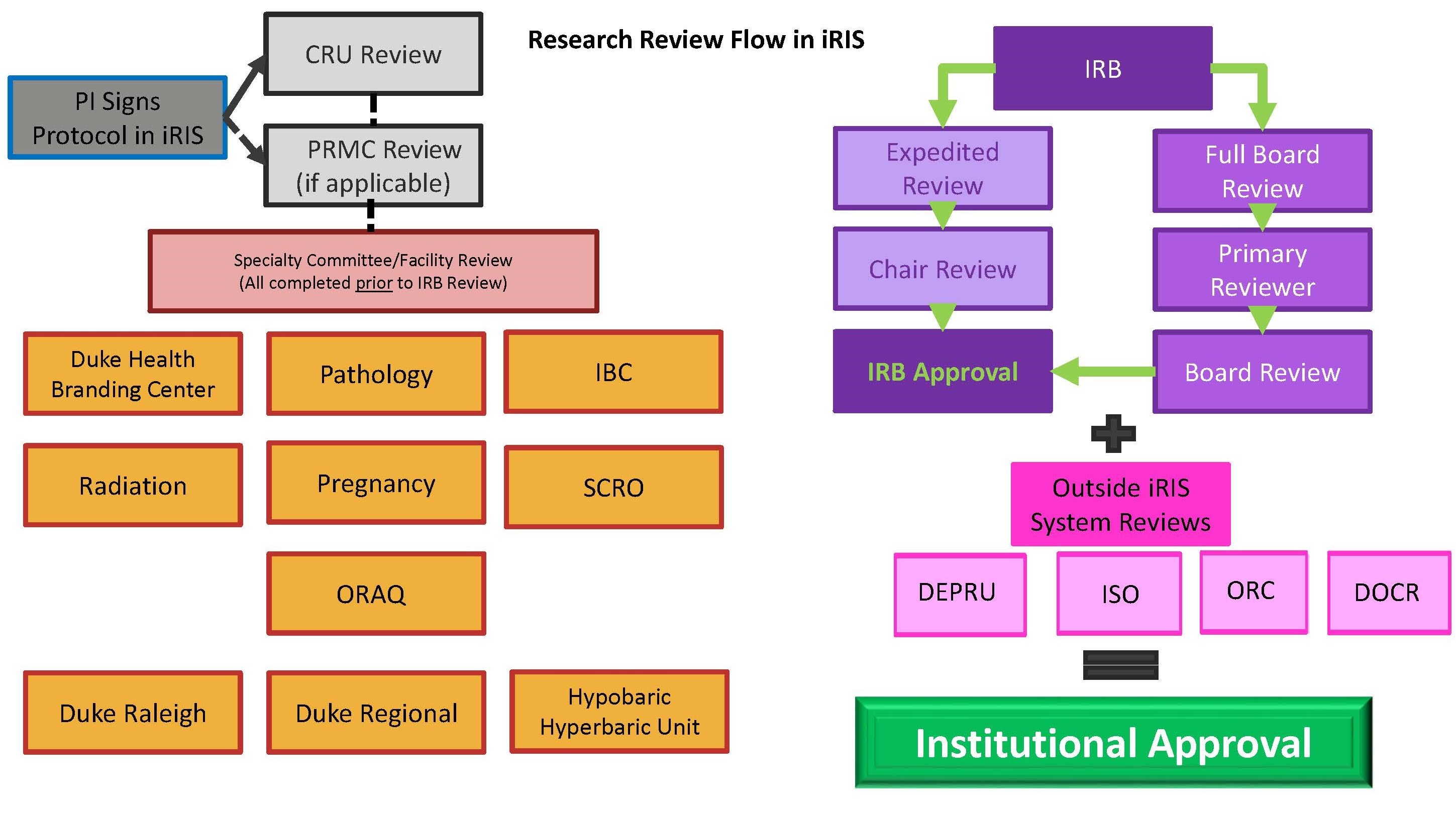
The flow diagram below shows the path a new study travels as it goes through review in the DUHS Human Research Protection Program (HRPP).
What Does the IRB Review?
The DUHS IRB reviews the following elements of research projects:
- Initial study submissions (new studies)
- Continuing reviews (renewals)
- Amendments
- Advertisements
- Adverse Events/Unanticipated Problems (safety events)
- Protocol Deviations and Violations
- Final Progress Reports
- Any study-related correspondence between DUHS personnel/sponsors/regulatory agencies
- Noncompliance issues
- Conflict-of-interest issues
Timeline for Renewal of IRB Approval
Maintaining continuous IRB approval for a study is the responsibility of the Principal Investigator and the study team. Please remember that if you allow your study's IRB approval to lapse, then you, the IRB, and Duke as an institution are considered to be out of compliance with federal regulations. To avoid having a study's IRB approval expire, please observe the following timeline.
Note the Expiration Date on your IRB Notification of Approval.
| Greater than 60 calendar days prior to Expiration Date: | Do not submit your renewal earlier than 60 days prior to Expiration Date. |
| 60 to 45 calendar days prior to Expiration Date: | DUHS IRB must receive your continuing review submission (renewal). |
| 30 calendar days prior to Expiration Date: | Your renewal submission is now considered late. You must e-mail or call your IRB Specialist and submit your renewal immediately. |
| 14 calendar days prior to Expiration Date: | If you have not submitted your renewal by the 14-day mark, you must submit a Deviation/Violation Report to the IRB. |
| DUHS IRB approval expires at 12 AM on the Expiration Date listed on the Notification of Approval. Therefore the Expiration Date represents the first date that the study is no longer approved. | At the time of expiration of IRB approval, all study activities, including ascertainment, recruitment, consent, data collection and data analysis, must stop. If you wish to continue certain activities directly related to subject safety, please e-mail Sharon Ellison, IRB Executive Director, to request permission to continue those activities until IRB approval is reinstated. |